Mechanistic Insights into the Alternating Copolymerization of Epoxides and Cyclic Anhydrides Using a (Salph)AlCl and Iminium Salt Catalytic System
TOC Graphic
Published In
Authors
Megan E. Fieser, Maria J. Sanford, Lauren A. Mitchell, Christine R. Dunbar, Mukunda Mandal, Nathan J. Van Zee, Devon M. Urness, Christopher J. Cramer*, Geoffrey W. Coates*, and William B. Tolman*
Citation
Fieser, M. E.; Sanford, M. J.; Mitchell, L. A.; Dunbar, C. R.; Mandal, M.; Van Zee, N. J.; Urness, D. M.; Cramer, C. J.; Coates, G. W.; Tolman, W. B. Mechanistic Insights into the Alternating Copolymerization of Epoxides and Cyclic Anhydrides Using a (Salph)AICI and Iminium Salt Catalytic System. J. Am. Chem. Soc. 2017, 139, 15222–15231. DOI: 10.1021/jacs.7b09079.
Express Summary
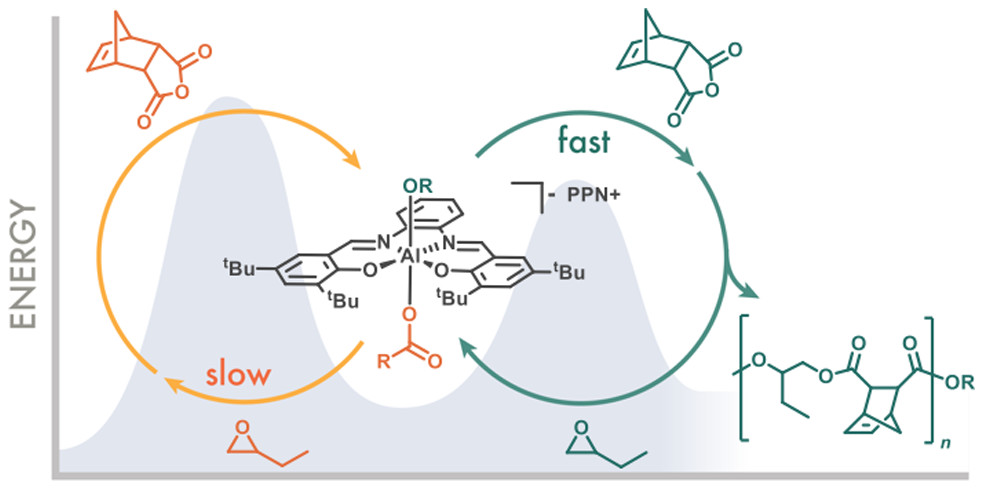
- Mechanistic studies of the copolymerization of 1-butene oxide and carbic anhydride using a (salph)AlCl/[PPN]Cl catalytic pair showed a first-order dependence of the polymerization rate on the epoxide and zero-order dependence on the cyclic anhydride.
- Model complexes showed that a mixed alkoxide/carboxylate aluminum intermediate preferentially opens cyclic anhydride over epoxide, and ring-opening of epoxide by an intermediate comprising multiple carboxylates was rate-determining.
- A mechanism involving two catalytic cycles is proposed where the copolymerization proceeds via intermediates with carboxylate ligation in common, avoiding a secondary cycle involving a bis-alkoxide species and explaining the lack of side reactions until the polymerization is complete.
Abstract
Mechanistic studies involving synergistic experiment and theory were performed on the perfectly alternating copolymerization of 1-butene oxide and carbic anhydride using a (salph)AlCl/[PPN]Cl catalytic pair. These studies showed a first-order dependence of the polymerization rate on the epoxide, a zero-order dependence on the cyclic anhydride, and a first-order dependence on the catalyst only if the two members of the catalytic pair are treated as a single unit. Studies of model complexes showed that a mixed alkoxide/carboxylate aluminum intermediate preferentially opens cyclic anhydride over epoxide. In addition, ring-opening of epoxide by an intermediate comprising multiple carboxylates was found to be rate-determining. On the basis of the experimental results and analysis by DFT calculations, a mechanism involving two catalytic cycles is proposed wherein the alternating copolymerization proceeds via intermediates that have carboxylate ligation in common, and a secondary cycle involving a bis-alkoxide species is avoided, thus explaining the lack of side reactions until the polymerization is complete.
This Article Contains These Key Ideas
- Idea-01.
- Hypothesis-01.
- Proof-01.
(Social Media Links!)
