Sterically Induced Ligand Framework Distortion Effects on Catalytic Cyclic Ester Polymerizations
TOC Graphic
Published In
Authors
Joahanna A. Macaranas, Anna M. Luke, Mukunda Mandal, Benjamin D. Neisen, Daniel J. Marell, Christopher J. Cramer*, and William B. Tolman*
Citation
Macaranas, J. A.; Luke, A. M.; Mandal, M.; Neisen, B. D.; Marell, D. J.; Cramer, C. J.; Tolman, W. B. Sterically Induced Ligand Framework Distortion Effects on Catalytic Cyclic Ester Polymerizations. Inorg. Chem. 2018, 57, 3451–3457. DOI: 10.1021/acs.inorgchem.8b00250.
Express Summary
- Effectiveness of aluminum salen complexes with o-adamantyl substituents in initiating the polymerization of ε-caprolactone was compared to that of similar salen catalysts having o-tbutyl substituents.
- DFT modeling indicates that the reactivity of the catalyst is influenced by both the length of the backbone linker and the presence of o-aryl substituents on the ligand.
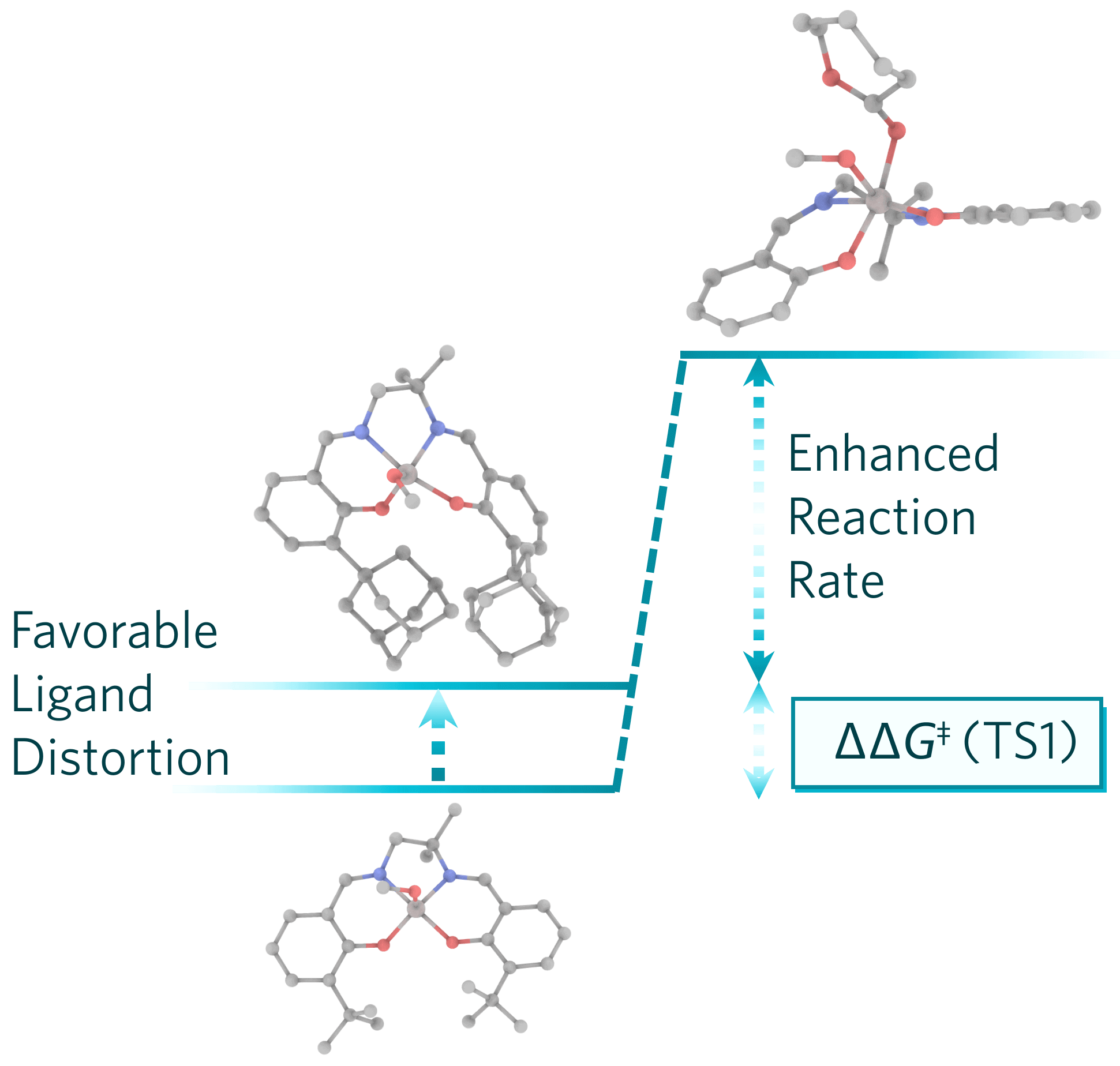
- Bulky o-substituents distorts the pre-catalyst geometry in a favorable way, making it easier to achieve the rate-limiting TS geometry, thus speeding up the reaction.
Abstract
Aluminum alkoxide complexes supported by salen ligands [salen = N,N′-bis(salicylaldimine)-2-methylpropane-1,2-diamine or N,N′-bis(salicylaldimine)-2,2-dimethylpropane-1,3-diamine] with o-adamantyl substituents have been synthesized and investigated for the polymerization of ε-caprolactone. Geometric analysis of the catalysts used for the reaction reveals the metal coordination geometries to be intermediate between square-pyramidal and trigonal-bipyramidal. A detailed kinetic study accompanied by density functional theory modeling of key mechanistic steps of the reaction suggest that, in addition to the length of the backbone linker, the o-aryl substituents have a significant impact on the catalyst’s reactivity. Bulky ortho substituents favorably distort the precatalyst geometry and thereby foster the achievement of the rate-limiting transition-state geometry at low energetic cost, thus accelerating the reaction.
This Article Contains These Key Ideas
- Idea-01.
- Hypothesis-01.
- Proof-01.
(Social Media Links!)
